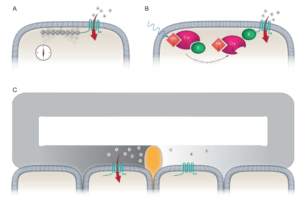
 Using maps, compasses, and sextants, mariners in the early 1500’s developed the first methods to navigate the open sea; heralding an age of exploration as humanity set sail for thehorizon. Yet long before this time evolution had equipped life on the planet with a biological global positioning system that was far superior to those early navigational tools – the Magnetic Sense. While there is unequivocal behavioural evidence demonstrating that this faculty exists, it is the least understood of all senses. The location of the primary sensors, the underlying biophysical mechanisms, and the neurological basis of the sense are unknown. Currently, there are three ideas that aim to explain how magnetosensation might work: (1) magnetite based magnetoreception; (2) a light sensitive radical pair based model; and (3) electromagnetic induction (See Figure).
Using maps, compasses, and sextants, mariners in the early 1500’s developed the first methods to navigate the open sea; heralding an age of exploration as humanity set sail for thehorizon. Yet long before this time evolution had equipped life on the planet with a biological global positioning system that was far superior to those early navigational tools – the Magnetic Sense. While there is unequivocal behavioural evidence demonstrating that this faculty exists, it is the least understood of all senses. The location of the primary sensors, the underlying biophysical mechanisms, and the neurological basis of the sense are unknown. Currently, there are three ideas that aim to explain how magnetosensation might work: (1) magnetite based magnetoreception; (2) a light sensitive radical pair based model; and (3) electromagnetic induction (See Figure).
Our goal is to identify the molecules, cells and circuits that underlie the magnetic sense in pigeons. To achieve this objective we employ an assay that assesses neuronal activation within the pigeon brain, following exposure to magnetic fields generated by Helmholtz coils. These experiments have confirmed that magnetic stimuli results in the activation of neurons in the vestibular nuclei, implicating the inner ear of pigeons in the magnetic sense. We have shown experimentally and by physical calculations that magnetic stimulation can induce electric fields in the pigeon semicircular canals that are within the physiological range of known electroreceptive systems. This in turn led to the discovery of a splice isoform of a voltage-gated calcium channel (CaV1.3) in the pigeon inner ear that has been shown to mediate electroreception in skates and sharks (Nimpf et al, Current Biology 2019). These data have led us to propose that pigeons detect magnetic fields by electromagnetic induction within the semicircular canals that is dependent on the presence of apically located voltage-gated cation channels in a population of electrosensory hair cells.
Current Projects:
Are hair cells the primary magnetosensors? Having established a physiologically relevant readout for the magnetic sense, we can now ask if hair cells are the primary sensors. To do so we are employing anatomical perturbations (e.g. hair cell ablation with antibiotics), which we expect will be superseded by genetic methods.
What neuronal circuits process magnetic information? To gain further insight into the underlying circuitry that processes magnetic information we have established an iDISCO clearing protocol for the pigeon brain that results in a translucent brain that can be stained with markers such as c-fos followed by light sheet microscopy (Fig. 2). We are employing this technology to identify the circuits that process magnetic information and to interrogate the underlying biophysical mechanisms.
How is magnetic information encoded in the avian brain? To address this question brain we have built a 2-photon microscope that permits in vivo calcium imaging while exposing pigeons to precise magnetic stimuli. Coupled with a genetically encoded calcium indicator (e.g GCaMP6) delivered by an adeno associated virus we are able to study which components of the magnetic field elicit neuronal activity (i.e. intensity, polarity and inclination) and how this information is integrated into existing neuronal networks. Are there magnetic place cells?
Figure 2: Image showing a whole pigeon brain prior to the iDISCO clearing protocol (left), after bleaching (middle), and following the removal of lipids (right). Coupled with immunostaining and light sheet microscopy this method allows us to globally assess neuronal activation in the pigeon brain.
Collaborators: Dr Jeremy Shaw (University of Western Australia), Dr Matthew Mason (University of Cambridge), Dr Michael Eisterer (Technical University Vienna).
Funded by: ERC Starting Grant – The Cellular and Molecular Basis of Magnetoreception, ERC Consolidator Grant – The Neurological Basis of the Magnetic sense.